To order this detailed 550+ page report, please visit this - https://www.rootsanalysis.com/reports/view_document/med-dev-regulatory/282.html
Key Inclusions
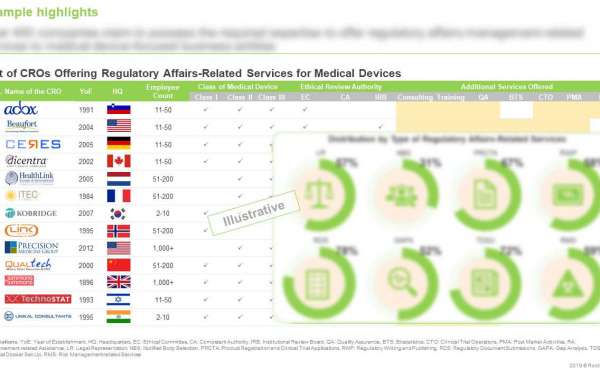
- A detailed review of the current market landscape of the medical devices regulatory affairs outsourcing market, featuring a list of over 400 CROs engaged in this domain, and detailed analysis based on a number of relevant parameters, such as year of establishment, size of employee base, geographical location, device class (class I, class II, and class III), type and size of clientele (medical device developers, medical device manufacturers, medical device research organizations, and others), types of services offered, ([A] regulatory management services (such as legal representation, notified body selection, project registration and clinical trial application, regulatory writing and publishing, regulatory document submission, product labelling related service, gap-analysis, technical dossier set-up, vigilance & medical device report, risk management-related services), [B] additional services (such as biostatistics, consulting, clinical operations, post-marketing activities, quality assurance, reimbursement, training)), region(s) of operation wherein the company is offering regulatory management services.
- A detailed review of the current Medical Device CRO market landscape of the medical devices regulatory affairs outsourcing market, featuring a list of over 400 CROs engaged in this domain.
- A detailed discussion on the need for regulatory review / oversight across different stages of the medical devices supply chain, with emphasis on the optimization of the supply chain using upcoming tools / technologies (such as artificial intelligence, big data analytical, blockchain, internet of things and others).
- An elaborate discussion on the various guidelines established by major regulatory bodies for medical device approval across North America (the US, Canada and Mexico), Europe (France, Germany, Italy, Spain, the UK and rest of Europe), Asia-Pacific and rest of the world (Australia, Brazil, China, India, Israel, Japan, New Zealand, Singapore, South Africa, South Korea, Taiwan, and Thailand). The report also features an insightful multi-dimensional, heat map analysis, featuring a comparison of the contemporary regulatory and reimbursement scenarios in key geographies across the globe.
- Elaborate profiles of popular players that specialize in offering end-to-end regulatory services for medical devices across key geographies (North America, Europe and Asia-Pacific). Each profile features a brief overview of the company, including information on company headquarters, year of establishment, number of employees, and therapeutic area expertise, financial information (if available), detailed description of service portfolio, and an informed future outlook.
- A benchmark analysis, highlighting the key focus areas of very small-sized, small-sized, mid-sized and large companies, comparing their existing capabilities within and beyond their respective peer groups, providing a means for stakeholders to identify ways to gain a competitive edge in the industry.
- An elaborate discussion on the various outsourcing business models adopted for regulatory affairs management, along with an insightful Harvey ball analysis of key considerations that need to be assessed by industry stakeholders while selecting a CRO partner.
- An analysis highlighting the key performance indicators used by sponsor companies to evaluate service providers that are active in the domain, based on information gathered via secondary research (for top-ten medical device players) and primary research.
- A survey analysis featuring inputs solicited from various experts who are directly / indirectly involved in providing regulatory affairs management services to medical device developers.
The report also features the likely distribution of the current and forecasted opportunity across important market segments, mentioned below:
- Medical Device Class
- Class I
- Class II
- Class III
- Therapeutic Area
- Cardiovascular Disorders
- CNS Disorders
- Metabolic Disorders
- Oncological Disorders
- Ophthalmological Disease
- Orthopedic Disorders
- Pain Disorders
- Respiratory Disorders
- Others
- Type of Regulatory Affairs Service
- Pharmacies GAP-Analysis
- Pharmacies Legal Representation
- Pharmacies Notified Body Selection
- Product Labelling-related Services
- Product Registration and Clinical Trial Applications
- Regulatory Document Submissions
- Regulatory Writing and Publishing
- Risk Management-related Services
- Technical Dossier Set-up
- Vigilance & Medical Device Report
- Key Geographical Regions
- North America
- Europe
- Asia-Pacific and Rest of the World
To request sample pages, please visit this - https://www.rootsanalysis.com/reports/282/request-sample.html
Key Questions Answered
- Who are the leading CROs offering regulatory affairs-management services for medical devices?
- What are differences in regulatory guidelines for medical device approval, across various geographies?
- What are the key performance indicators used by sponsors to evaluate potential service providers?
- What are the popular outsourcing models used by medical device companies for regulatory affairs-management purposes?
- What are the key challenges faced by medical device developers / manufacturers in terms of regulations related to medical device approvals?
- How is the current and future market opportunity likely to be distributed across key market segments?
You may also be interested in the following titles:
- Viral Vectors, Non-Viral Vectors and Gene Therapy Manufacturing Market (3rd Edition), 2019-2030
- Medical Device Labels Manufacturing Market, 2019-2030
- Medical Device Contract Manufacturing Market, 2019-2030
Contact Information
Roots Analysis Private Limited
Ben Johnson
+1 (415) 800 3415
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/company/roots-analysis/mycompany/
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/
Quora - https://rootsanalysisinsights.quora.com/
To order this detailed 550+ page report, please visit this - https://www.rootsanalysis.com/reports/view_document/med-dev-regulatory/282.html
Key Inclusions
- A detailed review of the current market landscape of the medical devices regulatory affairs outsourcing market, featuring a list of over 400 CROs engaged in this domain, and detailed analysis based on a number of relevant parameters, such as year of establishment, size of employee base, geographical location, device class (class I, class II, and class III), type and size of clientele (medical device developers, medical device manufacturers, medical device research organizations, and others), types of services offered, ([A] regulatory management services (such as legal representation, notified body selection, project registration and clinical trial application, regulatory writing and publishing, regulatory document submission, product labelling related service, gap-analysis, technical dossier set-up, vigilance & medical device report, risk management-related services), [B] additional services (such as biostatistics, consulting, clinical operations, post-marketing activities, quality assurance, reimbursement, training)), region(s) of operation wherein the company is offering regulatory management services.
- A detailed review of the current Medical Device CRO market landscape of the medical devices regulatory affairs outsourcing market, featuring a list of over 400 CROs engaged in this domain.
- A detailed discussion on the need for regulatory review / oversight across different stages of the medical devices supply chain, with emphasis on the optimization of the supply chain using upcoming tools / technologies (such as artificial intelligence, big data analytical, blockchain, internet of things and others).
- An elaborate discussion on the various guidelines established by major regulatory bodies for medical device approval across North America (the US, Canada and Mexico), Europe (France, Germany, Italy, Spain, the UK and rest of Europe), Asia-Pacific and rest of the world (Australia, Brazil, China, India, Israel, Japan, New Zealand, Singapore, South Africa, South Korea, Taiwan, and Thailand). The report also features an insightful multi-dimensional, heat map analysis, featuring a comparison of the contemporary regulatory and reimbursement scenarios in key geographies across the globe.
- Elaborate profiles of popular players that specialize in offering end-to-end regulatory services for medical devices across key geographies (North America, Europe and Asia-Pacific). Each profile features a brief overview of the company, including information on company headquarters, year of establishment, number of employees, and therapeutic area expertise, financial information (if available), detailed description of service portfolio, and an informed future outlook.
- A benchmark analysis, highlighting the key focus areas of very small-sized, small-sized, mid-sized and large companies, comparing their existing capabilities within and beyond their respective peer groups, providing a means for stakeholders to identify ways to gain a competitive edge in the industry.
- An elaborate discussion on the various outsourcing business models adopted for regulatory affairs management, along with an insightful Harvey ball analysis of key considerations that need to be assessed by industry stakeholders while selecting a CRO partner.
- An analysis highlighting the key performance indicators used by sponsor companies to evaluate service providers that are active in the domain, based on information gathered via secondary research (for top-ten medical device players) and primary research.
- A survey analysis featuring inputs solicited from various experts who are directly / indirectly involved in providing regulatory affairs management services to medical device developers.
The report also features the likely distribution of the current and forecasted opportunity across important market segments, mentioned below:
- Medical Device Class
- Class I
- Class II
- Class III
- Therapeutic Area
- Cardiovascular Disorders
- CNS Disorders
- Metabolic Disorders
- Oncological Disorders
- Ophthalmological Disease
- Orthopedic Disorders
- Pain Disorders
- Respiratory Disorders
- Others
- Type of Regulatory Affairs Service
- Pharmacies GAP-Analysis
- Pharmacies Legal Representation
- Pharmacies Notified Body Selection
- Product Labelling-related Services
- Product Registration and Clinical Trial Applications
- Regulatory Document Submissions
- Regulatory Writing and Publishing
- Risk Management-related Services
- Technical Dossier Set-up
- Vigilance & Medical Device Report
- Key Geographical Regions
- North America
- Europe
- Asia-Pacific and Rest of the World
To request sample pages, please visit this - https://www.rootsanalysis.com/reports/282/request-sample.html
Key Questions Answered
- Who are the leading CROs offering regulatory affairs-management services for medical devices?
- What are differences in regulatory guidelines for medical device approval, across various geographies?
- What are the key performance indicators used by sponsors to evaluate potential service providers?
- What are the popular outsourcing models used by medical device companies for regulatory affairs-management purposes?
- What are the key challenges faced by medical device developers / manufacturers in terms of regulations related to medical device approvals?
- How is the current and future market opportunity likely to be distributed across key market segments?
You may also be interested in the following titles:
- Viral Vectors, Non-Viral Vectors and Gene Therapy Manufacturing Market (3rd Edition), 2019-2030
- Medical Device Labels Manufacturing Market, 2019-2030
- Medical Device Contract Manufacturing Market, 2019-2030
Contact Information
Roots Analysis Private Limited
Ben Johnson
+1 (415) 800 3415
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/company/roots-analysis/mycompany/
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/